In a significant move toward improving malaria treatment and ensuring public health safety, the World Health Organization (WHO) has prequalified a diagnostic test to support the safer administration of treatments for Plasmodium vivax (P. vivax) malaria. This prequalification marks an important milestone in the fight against malaria, particularly the P. vivax strain, which remains a major public health challenge in tropical and subtropical regions around the world.
Understanding Malaria and P. vivax
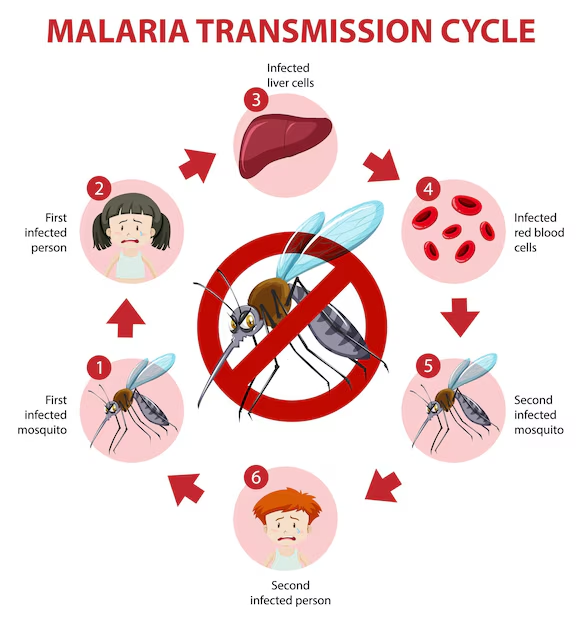
Malaria is a serious and often life-threatening disease caused by the Plasmodium parasites, which are transmitted to humans through the bite of an infected Anopheles mosquito. Despite substantial global efforts, malaria continues to be a major health burden in many parts of the world. In particular, the Plasmodium vivax species, one of the five species of Plasmodium that cause malaria in humans, remains the most widely distributed and represents a substantial challenge to malaria control and elimination efforts.
What Is Malaria?
Malaria is a vector-borne disease that is transmitted primarily through the bites of infected Anopheles mosquitoes. When an infected mosquito bites a human, it injects sporozoites—the parasite’s infective form—into the bloodstream. These sporozoites travel to the liver, where they mature, reproduce, and infect red blood cells, leading to the symptoms of malaria, including fever, chills, sweats, and fatigue. If left untreated, malaria can lead to severe complications such as anemia, organ failure, and even death.
There are five major species of Plasmodium that cause malaria in humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi. Of these, P. falciparum is the most dangerous and is responsible for the majority of malaria-related deaths, especially in sub-Saharan Africa. However, P. vivax remains the most widespread species and causes significant morbidity in tropical and subtropical regions, particularly in Southeast Asia, the Indian subcontinent, and parts of Africa and South America.
What Makes P. vivax Unique?
While P. falciparum is often associated with acute illness and rapid progression, P. vivax is unique in several ways. First, it is characterized by a relapsing nature that makes it distinct from other malaria species. This means that even after the symptoms of the initial infection subside, the parasite can remain dormant in the liver for months or even years before reactivating and causing a relapse of symptoms. This characteristic presents a major challenge for effective treatment and prevention of the disease.
Infected individuals typically experience a primary infection that may be relatively mild and sometimes overlooked. However, the dormant liver stage of P. vivax, called the hypnozoite, can remain undetected in the liver. The hypnozoites can reactivate at any time, causing a recurrence of symptoms. This is in contrast to P. falciparum, which does not have a dormant liver stage and tends to cause more acute and severe symptoms without the risk of relapses.
Moreover, P. vivax has a broader distribution compared to P. falciparum. While P. falciparum primarily affects sub-Saharan Africa, P. vivax is more common in Asia, Latin America, and parts of Africa. Its ability to survive and persist in diverse geographical regions makes it a persistent threat to global health.
The Life Cycle of P. vivax
The life cycle of P. vivax is similar to that of other Plasmodium species, with distinct stages in both the human host and the mosquito vector. The cycle begins when an infected female Anopheles mosquito takes a blood meal from a human, transmitting sporozoites into the bloodstream. These sporozoites travel to the liver, where they invade liver cells and mature into merozoites. This liver stage is where the parasite can remain dormant as hypnozoites, causing relapses in the future.
After the liver stage, the merozoites are released back into the bloodstream, where they invade red blood cells. Inside these cells, the parasites mature and reproduce. This stage is responsible for the characteristic symptoms of malaria, including fever, chills, and sweating, as the red blood cells burst, releasing new merozoites into the bloodstream to infect more cells.
In some cases, some of the merozoites differentiate into sexual forms (gametocytes), which can be ingested by a mosquito during a subsequent blood meal. The gametocytes mature inside the mosquito’s gut and undergo sexual reproduction, forming sporozoites that travel to the mosquito’s salivary glands, ready to infect the next human they bite. This completes the life cycle of the parasite.
Clinical Symptoms and Diagnosis of P. vivax Malaria
The clinical presentation of P. vivax malaria can vary, with some individuals experiencing mild symptoms and others suffering from more severe manifestations. The most common symptoms include fever, headache, fatigue, muscle pain, and chills. These symptoms typically follow a periodic pattern, occurring every 48 hours, as the parasite’s cycle of red blood cell invasion and rupture is synchronized with the body’s immune response.
Unlike P. falciparum, which can lead to severe disease and complications like cerebral malaria or acute kidney failure, P. vivax malaria is generally considered less lethal. However, it can still cause chronic illness, anemia, and long-term complications if not treated properly. The relapsing nature of P. vivax can also complicate treatment regimens, as individuals may experience repeated episodes of illness if dormant hypnozoites reactivate in the liver.
Diagnosing P. vivax malaria typically involves microscopic examination of blood samples, where the presence of the parasite in red blood cells is detected. In addition, rapid diagnostic tests (RDTs) have been developed to detect specific antigens produced by the parasite. These tests are critical in the field as they are easy to use and provide results in a matter of minutes, making them a valuable tool for diagnosing malaria in remote areas.
However, detecting dormant liver stages (hypnozoites) presents a challenge, as these stages are not detectable by standard diagnostic methods. This is why new diagnostic tools, such as the prequalified test by the World Health Organization (WHO), are essential for more accurate and early diagnosis, particularly for preventing relapses.
Treatment of P. vivax Malaria
The treatment of P. vivax malaria generally involves two drugs: a blood-stage drug (such as artemisinin-based combination therapies, or ACTs) and a liver-stage drug (such as primaquine or tafenoquine). The ACTs target the parasites in the bloodstream, while the liver-stage drugs are specifically designed to target hypnozoites and prevent relapses.
ACTs are the first-line treatment for P. vivax malaria, providing an effective way to kill the blood-stage parasites and reduce symptoms. However, in order to prevent relapses, the administration of a liver-stage drug is necessary. Primaquine has been used for this purpose for many years, but its use is limited by side effects such as hemolysis (destruction of red blood cells) in individuals with a deficiency in glucose-6-phosphate dehydrogenase (G6PD). G6PD deficiency is common in malaria-endemic areas, particularly in parts of Africa and Asia, and it can result in severe hemolytic anemia if individuals are given primaquine.
To address these challenges, new drugs such as tafenoquine have been developed. Tafenoquine is a single-dose treatment that can be used to prevent relapses, and it is effective for individuals with G6PD deficiency at lower doses. However, concerns about its cost and accessibility in low-resource settings remain.
The Challenges in Malaria Control
Despite progress in the fight against malaria, control efforts are hindered by several factors. One of the biggest challenges is the development of drug resistance. The increasing resistance of P. falciparum to artemisinin-based treatments is a growing concern in many regions, and this could potentially affect the effectiveness of treatments for P. vivax as well.
Another major challenge is the relapsing nature of P. vivax malaria, which complicates both diagnosis and treatment. The presence of dormant hypnozoites in the liver means that even after the initial infection is treated, relapses can occur months or even years later, requiring additional treatments to clear the infection completely.
Moreover, access to healthcare remains a significant barrier, particularly in remote and underserved areas where malaria is most prevalent. In many regions, health systems are ill-equipped to provide diagnostic services, appropriate treatment, or follow-up care, making it difficult to control the spread of the disease.
The Way Forward
The WHO has been at the forefront of efforts to combat malaria through the development of new diagnostic tools, medications, and vaccines. The prequalification of diagnostic tests for P. vivax malaria is a step toward improving diagnostic accuracy and ensuring that treatments are administered correctly to prevent relapses.
Moreover, ongoing research into vaccines, new antimalarial drugs, and vector control strategies will be essential in the fight against malaria. While significant progress has been made, the ultimate goal is to eradicate malaria globally and achieving this will require a coordinated effort at the international level.
The Importance of Diagnostic Tools in Combatting P. vivax Malaria
Malaria remains one of the most significant global health challenges, with the World Health Organization (WHO) estimating that there were 241 million cases of malaria worldwide in 2020, resulting in 627,000 deaths. Among the five species of Plasmodium that cause malaria in humans, P. vivax is the most widespread and continues to present a major challenge to global malaria control efforts. One of the key obstacles in controlling P. vivax malaria is the difficulty in detecting the parasite’s dormant liver stages, known as hypnozoites, which can cause relapses and complicate treatment.
In this context, diagnostic tools play a pivotal role in controlling the spread of malaria and ensuring timely and accurate treatment. However, the challenge with P. vivax lies in the unique nature of its life cycle. The parasite’s ability to remain dormant in the liver complicates both diagnosis and treatment. Early detection of P. vivax infection, particularly during its liver stage, is crucial in reducing morbidity, preventing relapses, and preventing the further spread of the disease. The introduction of a new diagnostic tool, recently prequalified by the WHO, promises to significantly enhance the accuracy of diagnosis and improve the management of P. vivax malaria.
Challenges in Diagnosing P. vivax Malaria
Malaria diagnosis typically relies on two primary methods: microscopic examination of blood samples and rapid diagnostic tests (RDTs). Microscopy involves examining a blood smear under a microscope to detect the presence of the parasite. This method is effective in identifying the parasite during the active blood stage of infection, when Plasmodium parasites are present in large numbers in red blood cells. However, microscopy has limitations. It can be time-consuming, requires skilled laboratory personnel, and is ineffective in detecting dormant liver stages, which are crucial for managing P. vivax malaria.
RDTs, on the other hand, offer a faster and simpler way to diagnose malaria, providing results within 15-20 minutes. RDTs detect specific antigens released by Plasmodium parasites into the bloodstream. However, these tests, while widely used in low-resource settings, also have limitations. RDTs are primarily designed to detect blood-stage infections, and as such, they cannot detect hypnozoites—the dormant liver stage of the parasite.
The inability to detect hypnozoites with standard diagnostic methods presents a significant problem for the treatment of P. vivax malaria. While primaquine and other liver-stage drugs are effective in preventing relapses, they can only be used properly once a diagnosis has been made. Without the ability to detect dormant parasites, healthcare providers risk misdiagnosis, leading to incomplete treatment and the potential for relapse.
Additionally, microscopic examination and RDTs are not always available in remote and resource-limited settings, where malaria burden is often highest. This lack of diagnostic tools further exacerbates the challenge of effectively treating and managing P. vivax malaria, particularly in regions where the disease is endemic.
The Role of Diagnostic Tools in Early Detection and Treatment
Early diagnosis of P. vivax malaria is critical to reducing morbidity, preventing complications, and halting the spread of the disease. Once malaria is diagnosed, treatment can begin immediately, preventing the parasite from multiplying and causing severe illness. Early diagnosis and prompt treatment are particularly essential for preventing severe malaria, which can lead to life-threatening complications, including anemia, organ failure, and even death.
However, the challenges of diagnosing P. vivax go beyond the limitations of standard diagnostic methods. The disease’s relapsing nature—caused by dormant hypnozoites—means that even after an initial infection is treated, there is a risk of future episodes. If left undiagnosed, the presence of hypnozoites can lead to multiple relapses over time, increasing the likelihood of chronic illness and further spread of the disease.
This is why the introduction of a new diagnostic tool is so important. The tool, which has been prequalified by the WHO, is designed to detect hypnozoites in the liver stage of infection, ensuring that P. vivax malaria can be diagnosed in its early stages, even before symptoms appear. By identifying dormant liver stages, healthcare providers can take preventive measures to avoid relapses and administer the appropriate treatment, such as primaquine or tafenoquine, which specifically target the liver-stage parasites.
The availability of such a tool would be a game-changer for malaria treatment, as it would enable healthcare providers to administer the correct treatment regimen from the outset, improving patient outcomes and reducing the risk of further complications. By identifying hypnozoites early, this diagnostic tool helps prevent future episodes of illness, minimizing the long-term impact of the disease.
The WHO Prequalified Diagnostic Test: A Step Forward in Malaria Control
The recent prequalification of a new diagnostic test for P. vivax malaria marks a significant milestone in the global fight against malaria. Prequalification is a rigorous process conducted by the WHO to ensure that diagnostic tools, medicines, and vaccines meet international standards of quality, safety, and effectiveness. The prequalification of this diagnostic test is a clear indication of its reliability and its potential to make a significant impact in malaria-endemic regions.
This diagnostic test has been specifically developed to detect hypnozoites—the dormant liver-stage parasites that are responsible for relapses in P. vivax malaria. By improving diagnostic accuracy, the test helps healthcare providers better identify P. vivax infections, even in their dormant phase, and tailor treatment regimens accordingly. This is particularly crucial in remote and rural areas, where access to advanced diagnostic resources is often limited.
According to the WHO, the new diagnostic test will enhance early detection of P. vivax malaria and improve the efficacy of treatment. In regions with limited access to healthcare facilities, this test will help bridge the gap between diagnosis and treatment, allowing healthcare providers to identify infections before they progress to more severe forms. The test’s ability to detect the dormant liver stage means that treatment can begin promptly, reducing the risk of relapses and ensuring that patients receive comprehensive care.
This tool is particularly valuable for high-burden malaria areas in Asia, Africa, and Latin America, where P. vivax remains a major public health threat. In these regions, access to advanced diagnostic resources is often scarce, and traditional diagnostic methods such as microscopy and RDTs are often the only available options. By providing a more accurate and comprehensive diagnostic solution, the prequalified test will help healthcare workers make informed decisions and deliver timely interventions, ultimately contributing to malaria control and elimination efforts.
The Impact on Global Malaria Elimination Efforts
The prequalification of the diagnostic tool aligns with the WHO’s global malaria strategy, which aims to reduce malaria cases and deaths by 90% by 2030. The strategy emphasizes the need for enhanced surveillance, early diagnosis, and targeted treatment to interrupt the transmission of malaria. By improving the accuracy of diagnosis, the new test will play a crucial role in achieving these ambitious goals.
Accurate diagnosis is critical to ensuring that patients receive the appropriate treatment. Malaria treatments, especially for P. vivax malaria, involve the use of two-drug combinations: one to treat the blood-stage parasites and another to target the dormant liver stage. Without accurate diagnosis, healthcare providers may fail to administer the correct combination of drugs, leading to incomplete treatment and the risk of relapses.
In addition to improving individual patient care, accurate diagnosis through the new test will contribute to more effective surveillance of malaria cases, providing valuable data to monitor the spread of the disease and inform public health interventions. This will help policymakers and public health experts better understand malaria transmission patterns and prioritize resources for the most affected areas.
Furthermore, enhanced diagnostics will help reduce the economic burden of malaria by ensuring that resources are used efficiently and effectively. Timely diagnosis and appropriate treatment will reduce the need for hospitalizations and prevent long-term complications associated with untreated or poorly treated malaria.
The Future of Malaria Diagnosis and Treatment
The introduction of this new diagnostic tool is just one step in the ongoing effort to combat malaria globally. While the prequalified test for P. vivax malaria is a significant advancement, ongoing research and innovation will be necessary to address the broader challenges posed by drug resistance, vector control, and access to care in malaria-endemic regions.
Future developments in malaria diagnostics may include molecular tests, genetic analysis, and point-of-care diagnostics, which can further enhance the ability to diagnose malaria and monitor treatment efficacy. These innovations, combined with effective treatments and vaccines, will be instrumental in the global effort to eradicate malaria.
The Role of the Prequalification Process in Enhancing Malaria Diagnosis
The prequalification process carried out by the World Health Organization (WHO) is a vital mechanism to ensure that medications, diagnostic tools, and vaccines meet the highest international standards for safety, efficacy, and quality. It is a cornerstone in the global effort to combat public health challenges like malaria, particularly in regions where access to healthcare is limited and where diseases like P. vivax malaria remain widespread. For countries that bear the brunt of malaria’s toll, the prequalification of diagnostic tests is more than just a regulatory step; it represents a critical move toward improving health outcomes in resource-poor settings.
With the recent prequalification of a diagnostic test for P. vivax malaria, the WHO has set the stage for better diagnosis, treatment, and prevention in areas heavily affected by malaria. In malaria-endemic countries, where access to advanced diagnostic infrastructure is often scarce, the prequalification process ensures that new diagnostic tools are safe, effective, and capable of addressing the specific needs of these regions. This process is a testament to the WHO’s dedication to equitable healthcare, aiming to provide low-resource countries with high-quality solutions to combat malaria and its devastating consequences.
What is the WHO Prequalification Process?
The WHO prequalification process is an extensive and comprehensive assessment that involves evaluating the safety, effectiveness, and quality of medications, vaccines, and diagnostic tools before they are recommended for use in low-resource environments. This process ensures that products meet stringent international standards and are fit for use in public health programs around the world. By undergoing the prequalification process, products undergo thorough scrutiny and testing to ensure they can withstand the challenges of real-world use—particularly in resource-poor settings.
Prequalification is essential because it provides global health organizations, including governments, donors, and healthcare providers, with the confidence that the tools and medicines they are using have been thoroughly evaluated and validated. This process is especially critical for countries with limited access to sophisticated diagnostic infrastructure, as it ensures that life-saving tools are both safe and effective. For instance, the new diagnostic test for P. vivax malaria, which has been prequalified by the WHO, has been evaluated against rigorous criteria that assess everything from accuracy to ease of use, ensuring that it meets the specific needs of low-resource settings.
The Importance of the Prequalification Process for Malaria Diagnosis
Malaria remains one of the deadliest infectious diseases globally, with P. vivax being the most widespread form of malaria, especially in Asia, Africa, and Latin America. The prequalification of diagnostic tools for this specific form of malaria is crucial, as early diagnosis and accurate treatment are essential to reducing morbidity, preventing relapses, and ultimately saving lives. Unfortunately, diagnosing P. vivax can be particularly difficult due to the parasite’s ability to enter a dormant liver stage called hypnozoites, which cannot be detected by traditional diagnostic methods like microscopy or rapid diagnostic tests (RDTs) designed for blood-stage parasites.
The challenge with P. vivax malaria is compounded by the fact that the hypnozoites can remain dormant in the liver for extended periods, only reactivating to cause a relapse of the infection. Standard diagnostic methods, like microscopy and RDTs, typically only detect the active blood-stage parasites and cannot identify these dormant liver stages. As a result, many patients in malaria-endemic countries may receive only partial treatment, potentially leading to relapses and continued transmission of the disease. Without a diagnostic tool capable of identifying both the active and dormant stages of the parasite, it is difficult to provide comprehensive care.
The prequalification of a new diagnostic tool that can detect hypnozoites is therefore a game-changer. By identifying the parasite during both its active and dormant stages, this new diagnostic tool ensures that patients receive the full course of treatment, preventing relapses and improving long-term health outcomes. The prequalification of this test validates its accuracy and reliability, which is essential for its widespread adoption in low-resource settings. It provides healthcare providers with the confidence that they can diagnose P. vivax malaria effectively and manage the disease with appropriate therapies, including liver-stage treatments like primaquine or tafenoquine, which target the hypnozoite.
Impact on Low-Resource Environments
One of the most significant aspects of the prequalification process is its focus on ensuring that diagnostic tools and medicines are accessible and effective in low-resource environments. In many malaria-endemic countries, healthcare infrastructure is limited, and access to advanced diagnostic tools may be restricted or non-existent. Traditional methods of diagnosing malaria, such as microscopy, often require specialized equipment and trained personnel, which are not always available in remote areas. Even rapid diagnostic tests (RDTs), while more accessible, often fail to detect dormant liver stages, which are particularly problematic in the treatment of P. vivax malaria.
In these settings, the prequalification of a new diagnostic test for P. vivax malaria is particularly important. It ensures that malaria-endemic countries—even those with limited resources—have access to a diagnostic tool that is both accurate and affordable. This test will enable healthcare workers to diagnose P. vivax infections early, even in the absence of advanced laboratory facilities, and provide patients with the necessary treatment to prevent relapses.
Moreover, the prequalification process ensures that the diagnostic tool is suitable for use in the field. Given that many malaria-endemic regions are located in remote or hard-to-reach areas, it is crucial that diagnostic tools be easy to use and require minimal infrastructure. The prequalified test for P. vivax malaria has been designed to meet these needs, offering a reliable and simple-to-use solution for diagnosing malaria in challenging settings. This is especially important in regions where malaria is endemic, and healthcare systems are often overwhelmed with high case loads and limited resources.
The introduction of this prequalified diagnostic tool will thus improve access to high-quality diagnostics, providing healthcare providers with the tools they need to make informed decisions and offer effective treatments. By improving diagnostic accuracy, the tool helps to prevent misdiagnosis, minimize errors, and ensure that patients receive appropriate care.
Strengthening Global Malaria Control Programs
The prequalification of the diagnostic test for P. vivax malaria is not just important for individual patient care—it also plays a key role in strengthening global malaria control programs. The WHO’s Global Malaria Strategy aims to reduce malaria cases and deaths by 90% by 2030, with a focus on early diagnosis, timely treatment, and effective prevention. For these goals to be realized, accurate and reliable diagnostic tools are essential.
The prequalification process ensures that the diagnostic test meets the rigorous standards required to be used effectively in global malaria programs. As donors, governments, and non-governmental organizations (NGOs) provide funding and resources for malaria control efforts, they can be confident that the prequalified diagnostic tools are of the highest quality and can be effectively used in real-world settings. This reduces the risk of inefficient resource allocation, helping malaria control programs maximize their impact and reach.
In addition to its impact on individual treatment, the prequalified diagnostic tool will also enhance surveillance efforts. By improving diagnostic accuracy, it will contribute to better data collection, allowing health authorities to monitor malaria transmission more effectively. This improved surveillance will help guide policy decisions, allowing countries to allocate resources more efficiently and target interventions where they are needed most.
Empowering Health Workers in Malaria-Endemic Countries
The introduction of prequalified diagnostic tools also empowers healthcare workers in malaria-endemic countries. In many low-resource regions, healthcare workers are often tasked with diagnosing and treating malaria without access to specialized training or advanced laboratory infrastructure. The prequalification process ensures that the diagnostic test is easy to use, even for workers with limited training.
Moreover, by providing healthcare workers with validated and reliable diagnostic tools, they are better equipped to make accurate diagnoses, reduce errors, and provide timely treatment. This not only improves patient outcomes but also strengthens the entire healthcare system in malaria-endemic countries.
The Test and Its Potential Impact
The diagnostic test, now prequalified by the WHO, can detect P. vivax malaria at the earliest stages, facilitating the prompt administration of effective treatments. The test is designed to identify parasites in the blood and liver stages, which is crucial for managing relapses associated with P. vivax. It works by detecting a unique biomarker that is present in the dormant liver stage of the parasite, allowing healthcare providers to detect the infection earlier and more accurately.
This advancement in diagnostics will have a profound impact on the treatment regimens for malaria, as it can guide healthcare professionals in making more informed decisions. This could result in reduced relapses, fewer complications, and improved patient outcomes.
Addressing Challenges in Malaria Treatment
One of the major challenges in malaria treatment is ensuring that individuals receive the correct medication for their specific type of infection. Misdiagnosis or delays in diagnosis can result in the use of ineffective treatments, which not only prolongs the infection but also worsens the disease. Moreover, with P. vivax malaria being prevalent in rural and underserved regions, where medical infrastructure is often limited, the availability of a reliable diagnostic tool becomes even more critical.
By prequalifying the test, the WHO is providing an essential resource for improving malaria control efforts. The prequalification of diagnostic tools and treatments plays a crucial role in ensuring equitable access to life-saving healthcare, particularly in areas where malaria is endemic.
The Future of Malaria Control
The prequalification of this new diagnostic test represents a promising step forward in the global fight against malaria. It complements the ongoing efforts by the WHO and its partners to reduce the burden of malaria through improved treatment options, vaccination programs, and vector control strategies. With the availability of accurate diagnostic tools, there is now a greater opportunity for targeted treatments, which could significantly reduce the number of cases and deaths caused by malaria.
The global health community, particularly in sub-Saharan Africa, Southeast Asia, and Latin America, can expect a positive impact on malaria control strategies, ultimately working toward the eradication of malaria. As new treatments, vaccines, and diagnostic tools continue to be developed, the role of the WHO in prequalifying these tools is vital to ensuring that they are safe, effective, and accessible to those who need them most.
Conclusion
The WHO’s prequalification of the diagnostic test for P. vivax malaria is a significant breakthrough in the ongoing efforts to combat malaria. By improving the accuracy and speed of diagnosis, this tool holds the potential to revolutionize the way malaria is diagnosed and treated, especially in resource-limited settings. With continued collaboration and innovation, the global community can look forward to achieving greater success in the fight against this deadly disease.
For more information on this groundbreaking development, you can visit the official WHO page on the news: WHO Prequalifies Diagnostic Test to Support Safer Administration of P. Vivax Malaria Treatments.
Final Thoughts
As the world continues to battle malaria, the integration of accurate diagnostic tools will be a critical pillar in the journey toward elimination. Innovation and global cooperation are key factors in addressing the complex challenges posed by malaria, and this prequalification by the WHO represents a major step toward ensuring that future generations live in a world free of this devastating disease.
Attribution:
This blog incorporates information from the World Health Organization (WHO). All credit for the official statement belongs to WHO.