Great Health Great Fitness
Unlocking the Secrets of Telomeres: A Groundbreaking Journey to Defy Aging and Embrace a Healthier, Vibrant Future

Aging has fascinated humankind for centuries, with individuals seeking ways to delay or even reverse the aging process. The scientific exploration of aging reveals numerous factors contributing to this natural phenomenon, one of which is the role of telomeres. These small, yet profoundly significant structures at the ends of chromosomes hold the key to understanding cellular aging and, by extension, human longevity.
Understanding Telomeres
Telomeres are repetitive nucleotide sequences at the ends of linear chromosomes that protect the genetic material during cell division. They act like the plastic tips of shoelaces, preventing the chromosomes from fraying or fusing with one another. In humans, telomeres consist of a repeated DNA sequence, TTAGGG, which can span several thousand base pairs.
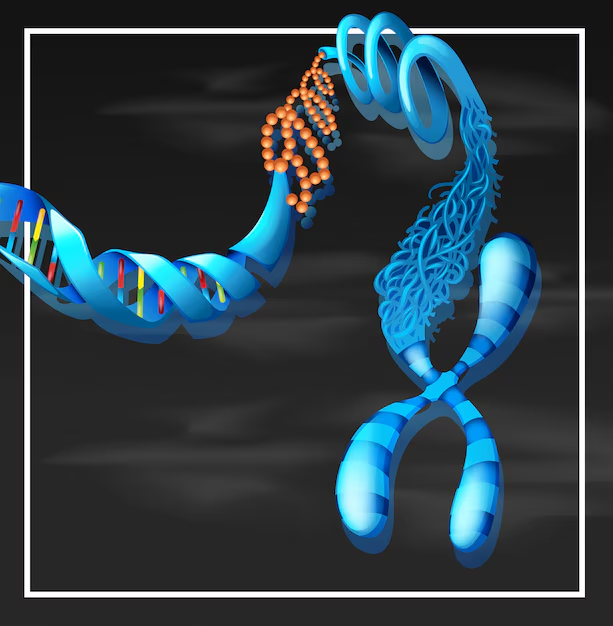
Every time a cell divides, the DNA replication process cannot fully copy the very end of the chromosome. This is known as the end-replication problem. Consequently, telomeres become progressively shorter with each division. Once telomeres reach a critically short length, cells can no longer divide effectively and enter a state called senescence, or they may undergo apoptosis, programmed cell death.
Telomeres and the Aging Process
The connection between telomeres and aging lies in the cumulative effects of telomere shortening over time. Shortened telomeres have been observed in various aging-related conditions and diseases, such as cardiovascular disease, neurodegenerative disorders, and immune dysfunction. Here are some critical aspects of this connection:
- Cellular Senescence: Telomere shortening triggers senescence, a state where cells stop dividing and secrete inflammatory molecules. This contributes to tissue dysfunction and the visible signs of aging.
- Stem Cell Exhaustion: Stem cells play a crucial role in tissue repair and regeneration. However, their capacity diminishes as telomeres shorten, leading to reduced regenerative potential and increased vulnerability to damage.
- Genomic Instability: Critically short telomeres can cause chromosome ends to fuse, leading to genomic instability. This increases the risk of mutations and cancer development.
- Oxidative Stress: Telomere shortening is exacerbated by oxidative stress, which damages DNA and accelerates the aging process. Oxidative stress is influenced by environmental factors such as pollution, smoking, and poor diet.
Telomerase: The Enzyme of Youth?
While telomeres naturally shorten with age, certain cells, such as germ cells, stem cells, and cancer cells, possess an enzyme called telomerase that can replenish telomeres. Telomerase adds repeated DNA sequences to the ends of chromosomes, counteracting telomere shortening and enabling cells to divide indefinitely.
Research into telomerase has revealed its dual nature:
- Anti-Aging Potential: Increasing telomerase activity in normal cells can prolong their lifespan and improve tissue function. Animal studies have shown that enhancing telomerase activity can extend lifespan and delay age-related diseases.
- Cancer Risk: Unregulated telomerase activity is a hallmark of cancer cells, allowing them to divide uncontrollably. This presents a challenge for using telomerase-based therapies in humans, as it could inadvertently promote tumor growth.
Telomeres and Lifestyle Factors
The rate of telomere shortening is not fixed and can be influenced by lifestyle choices and environmental factors. Numerous studies suggest that adopting a healthy lifestyle can slow telomere shortening and promote longevity. Key factors include:
- Diet: Consuming a diet rich in antioxidants, such as fruits, vegetables, and whole grains, can reduce oxidative stress and protect telomeres. Omega-3 fatty acids have also been associated with longer telomeres.
- Exercise: Regular physical activity has been linked to longer telomeres and improved cellular health. Moderate exercise, in particular, appears to have protective effects against telomere shortening.
- Stress Management: Chronic psychological stress has been shown to accelerate telomere shortening. Practices such as meditation, mindfulness, and yoga can help mitigate stress and its impact on cellular aging.
- Sleep: Adequate sleep is essential for cellular repair and maintaining telomere integrity. Poor sleep quality has been associated with shorter telomeres and increased risk of age-related diseases.
- Avoiding Harmful Habits: Smoking, excessive alcohol consumption, and exposure to pollutants can accelerate telomere shortening and contribute to premature aging.
Emerging Research and Future Directions
The field of telomere research is rapidly evolving, with scientists exploring innovative strategies to harness telomere biology for promoting health and longevity. Some promising areas of research include:
- Telomerase Activation Therapies: Developing drugs or interventions that safely activate telomerase in aging cells without increasing cancer risk.
- Gene Editing: Using techniques like CRISPR to repair or extend telomeres in specific cells, potentially reversing aspects of aging.
- Biomarkers of Aging: Telomere length is being investigated as a biomarker to assess biological age and the effectiveness of anti-aging interventions.
- Personalized Medicine: Understanding individual differences in telomere biology could lead to personalized approaches for preventing and treating age-related conditions.
Ethical and Societal Implications
While the potential to extend lifespan and improve health is exciting, it also raises significant ethical and societal questions:
- Access and Equity: Who will have access to telomere-based therapies, and how can we ensure they are not limited to the wealthy?
- Population Growth: Extending human lifespan could exacerbate challenges related to overpopulation and resource allocation.
- Definition of Aging: As our understanding of aging evolves, so too does the definition of what constitutes a “normal” lifespan.
Telomeres and Cellular Senescence
Cellular senescence refers to the irreversible cessation of cell division, a biological phenomenon that plays a critical role in maintaining organismal homeostasis. This process is frequently triggered by critically short telomeres, which are repetitive nucleotide sequences located at the ends of linear chromosomes. These telomeric sequences protect the genome from degradation, recombination, and end-to-end fusion. However, with each round of DNA replication, telomeres gradually shorten due to the inability of DNA polymerase to completely replicate the lagging strand, a limitation known as the “end-replication problem.”
When telomeres reach a critical length, they lose their protective function, exposing chromosomal ends and sending distress signals to the cell. This initiates a cascade of molecular events that activate pathways such as p53 and p16INK4a, pivotal tumor suppressor proteins. These pathways converge to halt the cell cycle, effectively rendering the cell incapable of further division. While this mechanism is protective against malignant transformation by preventing the propagation of damaged or potentially cancerous cells, it also contributes to the gradual accumulation of non-dividing, dysfunctional cells within tissues—a hallmark of aging.
The Role of Telomere Length in Cellular Function
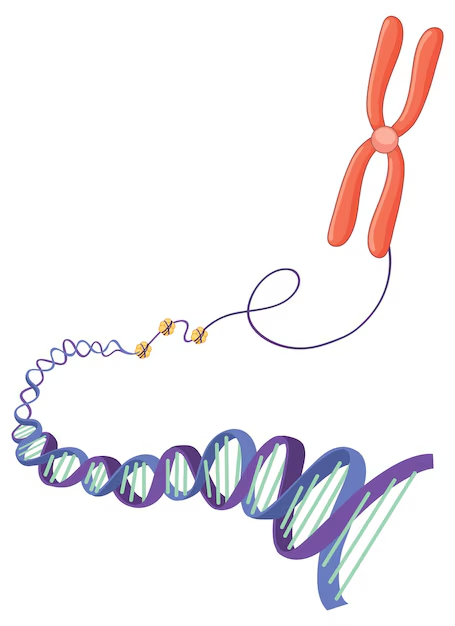
The correlation between telomere length and cellular function has been extensively studied, providing compelling evidence of its significance in determining cell and tissue health. Telomere attrition is not merely a marker of cellular aging but also a driving force behind it. Shortened telomeres impair the ability of cells to divide and replenish tissues, leading to reduced regenerative capacity and impaired tissue repair. This decline in cellular function is particularly evident in tissues with high turnover rates, such as the skin, blood, and gastrointestinal lining.
Furthermore, shortened telomeres are associated with increased vulnerability to a spectrum of age-related diseases, including:
- Cardiovascular Diseases: Short telomeres in endothelial and vascular smooth muscle cells contribute to arterial stiffness, endothelial dysfunction, and atherosclerosis.
- Diabetes: Telomere shortening in pancreatic beta cells and insulin-responsive tissues impairs glucose metabolism and insulin secretion.
- Neurodegenerative Disorders: Short telomeres in neurons and glial cells have been linked to diseases such as Alzheimer’s and Parkinson’s, exacerbating cognitive decline and neuronal death.
Conversely, maintaining telomere length is often associated with enhanced longevity and healthspan. Experimental evidence suggests that interventions aimed at preserving telomere integrity can delay the onset of age-related pathologies. For instance, activation of the enzyme telomerase, which adds telomeric repeats to chromosome ends, has shown promise in extending the lifespan of model organisms and improving cellular function.
Molecular Pathways in Telomere-Driven Senescence
The activation of molecular pathways in response to critically short telomeres is a sophisticated process that ensures cellular and genomic integrity. Two key pathways involved are:
- p53 Pathway: The tumor suppressor protein p53 plays a central role in the cellular response to telomere dysfunction. Upon sensing DNA damage or critically short telomeres, p53 is stabilized and activated, leading to the transcription of target genes that enforce cell cycle arrest, such as p21. This arrest prevents the proliferation of cells with damaged genomes, thereby acting as a safeguard against oncogenesis.
- p16INK4a Pathway: Another crucial pathway is mediated by p16INK4a, a cyclin-dependent kinase inhibitor. By inhibiting cyclin-dependent kinases 4 and 6 (CDK4/6), p16INK4a prevents the phosphorylation of the retinoblastoma (Rb) protein, maintaining it in an active state that represses E2F transcription factors and halts cell cycle progression.
Both pathways operate synergistically to impose a permanent state of cell cycle arrest, characterized by the upregulation of senescence-associated secretory phenotype (SASP) factors. SASP components, including pro-inflammatory cytokines, growth factors, and proteases, have both beneficial and detrimental effects. While they reinforce the senescent state and facilitate tissue remodeling, chronic SASP activity contributes to inflammation and tissue dysfunction, exacerbating aging and age-related diseases.
Telomere Shortening and Systemic Aging
At the organismal level, telomere shortening is a driver of systemic aging. It contributes to the decline in stem cell function and the progressive deterioration of tissue architecture and function. Stem cells, which are crucial for tissue regeneration, rely on intact telomeres for sustained self-renewal and differentiation. However, as telomeres shorten, stem cell exhaustion ensues, leading to reduced regenerative capacity and the onset of age-related phenotypes.
For example, hematopoietic stem cells (HSCs) with critically short telomeres exhibit diminished proliferative potential, resulting in impaired production of blood cells and compromised immune function. Similarly, mesenchymal stem cells (MSCs) with shortened telomeres show reduced differentiation into bone, cartilage, and adipose tissues, contributing to osteoporosis and other degenerative conditions.
Interventions to Preserve Telomere Length
Given the pivotal role of telomeres in cellular and organismal aging, various strategies have been explored to preserve telomere length and mitigate the effects of telomere attrition. These interventions include:
- Telomerase Activation: Telomerase, a ribonucleoprotein enzyme, counteracts telomere shortening by adding telomeric repeats to chromosome ends. Although telomerase is inactive in most somatic cells, its reactivation has shown promise in experimental models for delaying cellular senescence and extending lifespan. However, concerns about the potential oncogenic effects of telomerase activation remain a significant challenge.
- Lifestyle Modifications: Studies suggest that lifestyle factors such as regular exercise, a balanced diet rich in antioxidants, stress reduction, and adequate sleep can positively influence telomere length. For instance, physical activity has been associated with increased telomerase activity and reduced oxidative stress, both of which contribute to telomere preservation.
- Pharmacological Approaches: Small molecules and natural compounds that enhance telomerase activity or protect telomeres from damage are being actively investigated. Examples include TA-65, a telomerase activator derived from the Astragalus plant, and antioxidants that mitigate oxidative stress-induced telomere attrition.
- Gene Editing and Cellular Reprogramming: Advances in gene editing technologies, such as CRISPR-Cas9, offer the potential to target and repair telomere-related genetic defects. Additionally, cellular reprogramming techniques that convert somatic cells into induced pluripotent stem cells (iPSCs) restore telomere length, providing a rejuvenated cellular model for regenerative medicine.
Challenges and Future Directions
While significant progress has been made in understanding the role of telomeres in aging and disease, several challenges remain. One major hurdle is the complexity of telomere biology, which involves intricate interactions between genetic, epigenetic, and environmental factors. Additionally, the dual role of telomere shortening in preventing cancer and promoting aging underscores the need for balanced interventions that minimize risks while maximizing benefits.
Future research is likely to focus on:
- Developing targeted therapies that selectively activate telomerase in specific cell types without increasing cancer risk.
- Elucidating the interplay between telomeres and other hallmarks of aging, such as mitochondrial dysfunction and epigenetic alterations.
- Exploring the role of telomere length as a biomarker for aging and disease, enabling personalized interventions based on individual telomere dynamics.
The Enzyme Telomerase: A Guardian of Telomeres
Telomerase, a ribonucleoprotein enzyme, plays a pivotal role in counteracting telomere shortening, a process intimately linked to cellular aging and genomic stability. By adding telomeric repeats to the ends of chromosomes, telomerase maintains chromosomal integrity and prevents the loss of essential genetic information during cell division. This remarkable enzyme enhances the replicative potential of cells, allowing for sustained cell division under specific physiological conditions. Telomerase activity is highly dynamic and tightly regulated, with its most prominent expression observed in germ cells, stem cells, and certain white blood cells, where continuous division is critical for reproductive and immune functions. However, in contrast, most somatic cells exhibit minimal or no telomerase activity, leading to progressive telomere erosion and cellular senescence over time.
Telomeres and Their Biological Significance
Telomeres, the protective caps at the ends of linear chromosomes, are composed of repetitive DNA sequences (e.g., TTAGGG in humans) and associated proteins. They act as buffers, safeguarding the chromosome ends from being recognized as DNA breaks and preventing inappropriate repair mechanisms. Each round of DNA replication inevitably results in the loss of a small portion of telomeric DNA due to the “end-replication problem,” a limitation of DNA polymerases. Over successive cell divisions, telomeres gradually shorten, eventually triggering a DNA damage response that culminates in cellular senescence or apoptosis.
Telomerase counters this shortening by synthesizing new telomeric DNA, using its intrinsic RNA template (TERC) and catalytic protein subunit (TERT). This enzymatic activity is crucial in maintaining long-term genomic stability, particularly in highly proliferative cell types. Dysregulated telomere maintenance, however, can lead to profound biological consequences, ranging from premature aging syndromes to cancer progression.
Telomerase in Aging and Cellular Senescence
The progressive shortening of telomeres in somatic cells is widely regarded as a key hallmark of aging. As telomeres reach critically short lengths, cells enter a state of irreversible growth arrest known as replicative senescence, which is a potent barrier against uncontrolled cell proliferation. This mechanism, while protective against malignancy, contributes to age-related decline in tissue function, as senescent cells accumulate in various tissues and disrupt normal cellular environments through senescence-associated secretory phenotypes (SASP).
Conversely, the reactivation or upregulation of telomerase in somatic cells has been proposed as a potential therapeutic strategy to delay aging and promote tissue regeneration. Enhancing telomerase activity could theoretically restore telomere length, rejuvenating aged cells and improving their functional capacity. For instance, experimental studies involving telomerase gene therapy in animal models have demonstrated extended lifespan and improved healthspan. However, such approaches are fraught with challenges, particularly the risk of oncogenesis due to uncontrolled cell proliferation. Thus, while the prospect of utilizing telomerase as an “anti-aging enzyme” is tantalizing, its clinical application requires careful consideration of safety and efficacy.
Telomerase and Cancer: A Double-Edged Sword
One of the most intriguing and paradoxical aspects of telomerase biology lies in its role in cancer progression. Unlike normal somatic cells, which exhibit low or negligible telomerase activity, approximately 85-90% of cancer cells reactivate telomerase, enabling them to maintain telomere length and achieve limitless replicative potential. This reactivation is a critical step in cellular immortalization, allowing cancer cells to bypass senescence and apoptosis despite accumulating extensive genetic mutations.
The activation of telomerase in cancer cells often results from TERT promoter mutations, gene amplification, or epigenetic modifications. These alterations confer a selective advantage to malignant cells, promoting tumorigenesis and metastasis. Consequently, telomerase represents an attractive target for anticancer therapies, with several approaches under investigation:
- Telomerase Inhibitors: Small molecules, antisense oligonucleotides, or immunotherapies designed to suppress telomerase activity or expression in cancer cells.
- Telomere Targeting: Agents that disrupt the protective structure of telomeres, rendering cancer cells more susceptible to genomic instability.
- Oncolytic Viruses: Engineered viruses that selectively replicate in telomerase-positive cells, leading to their destruction.
While these strategies hold promise, their clinical translation has been challenging, as telomerase inhibition must be sufficiently selective to avoid adverse effects on normal regenerative tissues with physiological telomerase activity.
Therapeutic Potential of Telomerase Activation
Beyond its implications in cancer, telomerase activation holds significant potential for addressing degenerative diseases and conditions associated with telomere dysfunction. Disorders such as dyskeratosis congenita, idiopathic pulmonary fibrosis, and aplastic anemia are characterized by critically short telomeres and impaired tissue maintenance. In such contexts, enhancing telomerase activity could restore telomere length and alleviate disease manifestations.
Moreover, emerging evidence suggests that telomerase plays broader roles beyond telomere maintenance. For instance, TERT has been implicated in modulating mitochondrial function, oxidative stress responses, and cellular signaling pathways, highlighting its multifaceted contributions to cellular homeostasis. Harnessing these non-canonical functions of telomerase could open new avenues for therapeutic development.
Challenges and Ethical Considerations
Despite the exciting prospects of telomerase-based therapies, several challenges and ethical considerations must be addressed:
- Oncogenic Risks: The potential for telomerase activation to promote cancer remains a significant concern. Developing strategies to achieve precise and controlled telomerase modulation is critical for minimizing these risks.
- Delivery Mechanisms: Efficient and targeted delivery of telomerase activators or inhibitors to specific tissues or cell types poses technical hurdles.
- Long-Term Effects: The long-term consequences of manipulating telomerase activity are not fully understood, necessitating extensive preclinical and clinical studies.
- Ethical Implications: The possibility of extending human lifespan or enhancing regenerative capacity raises ethical questions regarding resource allocation, equity, and societal impacts.
Telomeres and Aging: Insights from Research
The relationship between telomeres and aging is a rapidly advancing field that has captured the interest of scientists worldwide. Telomeres, the protective caps at the ends of chromosomes, play a critical role in maintaining genetic stability. Their length and integrity are directly influenced by various lifestyle factors, genetic predispositions, and epigenetic modifications. Below is an in-depth exploration of these aspects and their implications for aging.
Lifestyle Factors and Telomere Health

Lifestyle choices have a profound impact on telomere length and, consequently, aging and overall health. Various studies have highlighted how unhealthy behaviors can accelerate telomere shortening, while positive lifestyle changes may help maintain or even lengthen telomeres.
Negative Lifestyle Factors
- Chronic Stress:
- Persistent stress triggers the release of cortisol, a stress hormone that can cause inflammation and oxidative stress, both of which contribute to telomere attrition.
- Individuals experiencing prolonged emotional or physical stress often show significantly shorter telomeres compared to their less-stressed counterparts.
- Poor Diet:
- Diets high in processed foods, saturated fats, and added sugars promote oxidative damage to cells, including telomeres.
- Low intake of micronutrients, such as vitamins C and E, which act as antioxidants, exacerbates telomere shortening.
- Smoking:
- Cigarette smoke is a significant source of oxidative stress and inflammation. Smokers exhibit shorter telomeres, correlating with an increased risk of age-related diseases.
- Sedentary Lifestyle:
- Physical inactivity is associated with shorter telomeres and increased susceptibility to chronic diseases like diabetes and cardiovascular conditions.
Positive Lifestyle Practices
- Regular Exercise:
- Aerobic and moderate physical activity stimulate the production of antioxidant enzymes, reducing oxidative stress and protecting telomeres.
- Activities such as walking, cycling, and yoga have been linked to longer telomeres in various studies.
- Balanced Diet Rich in Antioxidants:
- Diets emphasizing fruits, vegetables, whole grains, and lean proteins are rich in antioxidants and anti-inflammatory compounds, safeguarding telomeres.
- Foods like blueberries, nuts, fatty fish, and leafy greens contribute to telomere maintenance by neutralizing free radicals.
- Stress-Reducing Activities:
- Practices such as mindfulness meditation, tai chi, and deep breathing exercises reduce cortisol levels and improve telomere stability.
- Adequate Sleep:
- Sufficient restorative sleep enhances cellular repair mechanisms and reduces inflammation, indirectly supporting telomere health.
Genetic Influence
While lifestyle plays a pivotal role in telomere dynamics, genetic predispositions also significantly affect telomere length and maintenance. Specific genes regulate telomerase activity, the enzyme responsible for elongating telomeres.
- TERT (Telomerase Reverse Transcriptase):
- TERT encodes the catalytic subunit of telomerase, which adds repetitive nucleotide sequences to the ends of telomeres.
- Variants in the TERT gene can either enhance or impair telomerase activity, influencing cellular aging and the risk of diseases like cancer.
- TERC (Telomerase RNA Component):
- TERC is another critical component of the telomerase complex, serving as a template for telomere elongation.
- Genetic variations in TERC have been associated with differences in telomere length and susceptibility to conditions like aplastic anemia and idiopathic pulmonary fibrosis.
- Shelterin Complex Genes:
- Proteins such as TRF1, TRF2, and POT1, which are part of the shelterin complex, protect telomeres from being recognized as DNA damage.
- Mutations in these genes can lead to telomere dysfunction and genomic instability.
Epigenetic Modifications
Epigenetics, which involves heritable changes in gene expression without alterations in the DNA sequence, has emerged as a critical factor in telomere biology. Epigenetic modifications influence how genes related to telomere maintenance are expressed, affecting telomere length and function.
- DNA Methylation:
- Methylation of CpG islands near telomere-related genes can either upregulate or downregulate their activity.
- Hypomethylation at telomeres has been linked to chromosomal instability, while hypermethylation can silence essential repair genes.
- Histone Modifications:
- Modifications such as acetylation, methylation, and phosphorylation of histones influence the chromatin structure surrounding telomeres.
- Altered histone patterns can lead to either enhanced protection or exposure of telomeric DNA, affecting its stability.
- Non-coding RNAs:
- Telomeric repeat-containing RNA (TERRA) is a type of long non-coding RNA that regulates telomere length and function.
- Dysregulation of TERRA has been implicated in age-related diseases and cellular senescence.
Animal Studies
Research in model organisms has provided valuable insights into the role of telomeres and telomerase in aging and disease. Animal studies offer a controlled environment to manipulate telomere biology and observe its effects on lifespan and healthspan.
- Telomerase Activation and Lifespan Extension:
- Studies in mice have shown that enhancing telomerase activity can extend their lifespan.
- For example, genetically engineered mice with elevated telomerase levels exhibit delayed onset of age-related diseases, improved organ function, and extended lifespan.
- Telomere Dysfunction and Disease Models:
- Telomerase knockout mice, which lack functional telomerase, develop symptoms resembling premature aging, such as osteoporosis, heart disease, and immune dysfunction.
- These models underscore the critical role of telomerase in preventing age-related decline.
- Limitations in Translatability to Humans:
- While findings in animal models are promising, significant differences exist between human and rodent telomere biology.
- Mice have much longer telomeres and higher telomerase activity compared to humans, making direct extrapolation of results challenging.
- Therapeutic Interventions:
- Potential therapies based on telomerase activation are being investigated. However, the risk of promoting uncontrolled cell proliferation and cancer remains a major concern.
- Future research aims to fine-tune telomerase-based therapies to maximize benefits while minimizing risks.
Telomeres and Longevity: Beyond Cellular Aging

Telomeres, the protective caps at the ends of eukaryotic chromosomes, play a crucial role in maintaining genomic stability. These repetitive DNA sequences, primarily composed of TTAGGG repeats in humans, act as buffers against the erosion of coding DNA during cell division. However, their significance transcends cellular-level mechanisms, influencing systemic aging, longevity, and overall health.
The Dual Role of Telomeres in Aging
The length and integrity of telomeres are pivotal in determining the replicative capacity of cells. With each cell division, telomeres gradually shorten due to the end-replication problem inherent in DNA replication. Once telomeres reach a critical length, cells enter a state of senescence or undergo programmed cell death (apoptosis). While these mechanisms prevent genomic instability, they also contribute to tissue aging and dysfunction.
Telomere Length and Systemic Aging
Beyond cellular aging, telomere length has systemic implications:
- Immune Function: Long telomeres correlate with robust immune responses, enabling the body to combat infections and diseases effectively. Conversely, short telomeres are associated with immunosenescence—the gradual deterioration of the immune system with age.
- Inflammation: Chronic inflammation, often termed “inflammaging,” is a hallmark of aging. Short telomeres exacerbate inflammatory responses by inducing senescence-associated secretory phenotype (SASP), wherein senescent cells secrete pro-inflammatory cytokines and chemokines.
- Age-Related Diseases: Epidemiological studies link short telomeres to a higher risk of atherosclerosis, hypertension, and Alzheimer’s disease. These conditions not only reduce lifespan but also impair the quality of life in aging populations.
Stem Cells and Tissue Regeneration
Stem cells are indispensable for tissue repair and regeneration. However, their replicative potential is finite, governed by telomere dynamics. As telomeres shorten, stem cells lose their ability to proliferate effectively, leading to several age-related phenotypes:
- Frailty: Reduced stem cell function impairs the regeneration of skeletal muscle and bone, contributing to physical weakness and increased vulnerability to injuries.
- Reduced Muscle Mass: Short telomeres limit the ability of satellite cells (muscle stem cells) to repair and regenerate muscle tissue, resulting in sarcopenia.
- Impaired Wound Healing: Telomere attrition in skin fibroblasts and keratinocytes compromises the body’s ability to repair wounds efficiently.
Telomerase: The Enzyme of Immortality?
Telomerase, a ribonucleoprotein enzyme, counters telomere shortening by adding TTAGGG repeats to the ends of chromosomes. While most somatic cells exhibit low telomerase activity, certain cell types, such as germ cells, stem cells, and cancer cells, maintain their telomeres through high telomerase expression. This enzyme has been a focal point in aging and cancer research:
- Potential in Anti-Aging Therapies: Activating telomerase in somatic cells has been proposed as a strategy to delay aging and extend lifespan. However, this approach carries the risk of uncontrolled cell proliferation and cancer.
- Cancer and Telomeres: Cancer cells exploit telomerase to achieve replicative immortality, highlighting the dual-edged nature of telomere elongation.
Lifestyle Factors Influencing Telomere Dynamics
While genetics play a significant role in determining telomere length, lifestyle factors also impact telomere attrition. Evidence suggests that healthy habits can preserve telomere length and promote longevity:
- Physical Activity: Regular exercise is associated with longer telomeres and reduced markers of oxidative stress and inflammation.
- Diet: A diet rich in antioxidants, such as fruits, vegetables, and whole grains, helps mitigate oxidative damage to telomeres.
- Stress Management: Chronic psychological stress accelerates telomere shortening by increasing cortisol levels and oxidative stress. Mindfulness practices and stress reduction techniques can counteract this effect.
- Sleep: Poor sleep quality and duration have been linked to shorter telomeres, emphasizing the importance of restorative sleep for cellular health.
Emerging Research and Therapeutic Interventions
Recent advances in telomere biology have opened new avenues for therapeutic interventions aimed at combating aging and age-related diseases:
- Gene Editing: Techniques like CRISPR-Cas9 offer the potential to directly manipulate telomere sequences or enhance telomerase activity in specific cell types.
- Small Molecule Activators: Compounds that upregulate telomerase expression or stabilize telomeres are being explored as potential anti-aging therapies.
- Senolytics: Drugs targeting senescent cells aim to mitigate the deleterious effects of SASP and improve tissue function.
Ethical and Practical Considerations
While telomere-based interventions hold promise, they also raise ethical and practical concerns:
- Equity: Access to advanced therapies may exacerbate existing health disparities.
- Safety: Prolonging telomere length could inadvertently increase the risk of malignancies.
- Natural Limits: Extending lifespan beyond natural limits poses philosophical and societal questions about the nature of aging and mortality.
Therapeutic Interventions Targeting Telomeres
Telomeres, the protective caps at the ends of chromosomes, are fundamental to cellular aging and longevity. As cells divide, telomeres progressively shorten, leading to cellular senescence or apoptosis when critical length thresholds are reached. This process is central to aging and age-related diseases. Various therapeutic strategies aim to modulate telomere dynamics, offering potential avenues to delay aging, improve healthspan, and combat diseases associated with telomere dysfunction. This document elaborates on the proposed interventions, emphasizing the pivotal role of telomerase activation, lifestyle modifications, pharmacological interventions, and epigenetic therapies.
1. Telomerase Activation
Telomerase, an enzyme that extends telomeres by adding repetitive nucleotide sequences, plays a crucial role in maintaining chromosomal integrity. While active in germline and certain stem cells, telomerase is largely inactive in somatic cells, leading to telomere attrition over time. Reactivating telomerase in somatic cells has emerged as a promising strategy to counteract aging and its associated ailments.
Mechanisms and Approaches
- Gene Therapy: Gene-editing technologies, such as CRISPR-Cas9, offer precise methods to introduce or enhance telomerase expression in targeted cells. Early studies demonstrate improved cellular rejuvenation and delayed senescence.
- Small Molecule Activators: Compounds like TA-65 have been identified to upregulate telomerase activity. Preliminary findings suggest these molecules can extend telomeres and enhance cellular function.
- Peptide-Based Interventions: Telomerase-activating peptides are being designed to specifically bind and activate the enzyme, thereby mitigating telomere shortening.
Challenges
Despite its potential, telomerase activation poses significant challenges. Unregulated telomerase activity is associated with increased cancer risk, as cancer cells exploit this mechanism for unchecked proliferation. Balancing efficacy and safety remain a critical concern, necessitating targeted delivery systems and stringent monitoring.
2. Lifestyle Modifications
Lifestyle interventions, though non-invasive, exert profound effects on telomere dynamics. These modifications target underlying mechanisms of telomere attrition, such as oxidative stress and inflammation, emphasizing prevention and maintenance over restoration.
Dietary Interventions
- Antioxidant-Rich Diets: Foods high in antioxidants, such as fruits, vegetables, and nuts, help neutralize reactive oxygen species (ROS), reducing oxidative damage to telomeres.
- Mediterranean Diet: Rich in whole grains, healthy fats, and lean proteins, this diet is associated with longer telomeres and reduced incidence of chronic diseases.
- Caloric Restriction: Controlled reduction in calorie intake, without malnutrition, has been shown to preserve telomere length and enhance lifespan in preclinical models.
Physical Activity
- Moderate Exercise: Regular aerobic activities, such as walking, cycling, and swimming, correlate with longer telomeres and reduced markers of biological aging.
- Avoiding Overtraining: While moderate exercise is beneficial, excessive physical stress can elevate oxidative damage, negating telomere benefits.
Stress Management
- Mindfulness Practices: Meditation and yoga have demonstrated reductions in stress-induced telomere shortening by modulating cortisol levels.
- Social Connections: Strong social networks and emotional well-being are linked to healthier telomere dynamics.
Sleep Optimization
- Adequate and quality sleep supports cellular repair mechanisms, indirectly protecting telomere integrity.
3. Pharmacological Interventions
Pharmacological approaches target molecular pathways implicated in telomere maintenance, offering a direct means to preserve telomere length and enhance cellular function.
Antioxidants and ROS Modulators
- Vitamin C and E: These vitamins reduce oxidative damage, preventing telomere attrition in various cell types.
- Mitochondria-Targeted Antioxidants: Molecules like MitoQ and SkQ1 specifically address mitochondrial ROS, a major contributor to telomere damage.
Anti-Inflammatory Agents
- NSAIDs: Non-steroidal anti-inflammatory drugs (e.g., aspirin) reduce systemic inflammation, indirectly preserving telomere length.
- Cytokine Modulators: Drugs targeting pro-inflammatory cytokines (e.g., IL-6 and TNF-α inhibitors) help reduce telomere-associated inflammation.
Senolytics
- Senescence-Targeting Drugs: These agents selectively eliminate senescent cells, reducing inflammation and promoting tissue regeneration. Examples include dasatinib and quercetin.
Telomerase Modulators
- Small molecules designed to upregulate telomerase expression directly or through upstream signaling pathways represent a focused pharmacological avenue.
4. Epigenetic Therapies
Epigenetic modifications, such as DNA methylation and histone acetylation, influence telomere maintenance and expression of telomerase. Interventions targeting these modifications aim to reverse detrimental changes associated with aging.
DNA Methylation Modulators
- DNMT Inhibitors: Drugs that inhibit DNA methyltransferases (e.g., azacitidine) can reactivate silenced genes associated with telomere maintenance.
Histone Modifications
- HDAC Inhibitors: Histone deacetylase inhibitors promote chromatin relaxation, enhancing the accessibility of telomerase-related genes.
RNA-Based Therapies
- siRNA and Antisense Oligonucleotides: These molecules target specific RNA transcripts to modulate telomerase activity and telomere dynamics.
Integration and Future Directions
While individual strategies hold promise, a combined approach leveraging multiple interventions may offer the most effective outcomes. Integrating telomerase activation, lifestyle modifications, pharmacological agents, and epigenetic therapies can address telomere dynamics comprehensively. However, future research must prioritize:
- Safety: Mitigating risks, especially cancer, through precision targeting.
- Long-Term Efficacy: Ensuring sustained benefits without adverse effects.
- Accessibility: Making therapies cost-effective and widely available.
The Role of Telomeres in Aging
Telomeres are complex structures consisting of repetitive DNA sequences at the ends of chromosomes. Their primary function is to protect the chromosome from degradation, preventing the loss of important genetic information during cell division. Each time a cell divides, the telomeres shorten, eventually reaching a point where they can no longer protect the chromosomes effectively. This progressive telomere attrition is closely linked to cellular senescence, a state in which cells lose their ability to divide and function properly.
The shortening of telomeres is considered one of the primary drivers of aging at the cellular level. As telomeres become critically short, cells either enter a permanent state of dormancy (senescence) or undergo programmed cell death (apoptosis). This process contributes to tissue dysfunction, organ aging, and the development of age-related diseases such as cardiovascular disease, diabetes, and neurodegenerative disorders.
Moreover, telomere length has been associated with the aging of the organism as a whole. Studies have shown that individuals with shorter telomeres tend to experience more rapid aging and have an increased risk of age-related diseases. Consequently, telomeres are considered a potential target for interventions designed to slow or reverse the aging process.
Telomere Extension and Anti-Aging Therapies
The potential to manipulate telomeres for anti-aging purposes is a captivating area of research. If we could somehow extend telomeres or prevent their shortening, it might be possible to delay the onset of aging and associated diseases. There are several proposed strategies to achieve telomere extension, including genetic manipulation, telomerase activation, and the use of small molecules.
- Telomerase Activation: Telomerase is an enzyme that adds DNA sequences to the ends of telomeres, effectively counteracting their shortening. In most somatic cells, telomerase activity is minimal or absent, which is why telomeres shorten with each cell division. However, certain types of cells, such as germ cells and stem cells, express telomerase to maintain telomere length and enable continuous division. One promising approach in anti-aging research is the activation of telomerase in somatic cells. Studies in model organisms, such as mice, have shown that telomerase activation can extend lifespan and improve age-related health. However, the risk of cancer arises when cells with extended telomeres begin dividing uncontrollably.
- Gene Editing and CRISPR: With the advent of CRISPR-Cas9 gene editing technology, researchers can now precisely modify genetic material to target telomere-related genes. By editing genes that regulate telomere maintenance, such as those responsible for telomerase expression, it may be possible to prolong telomere length and delay aging. While this technology holds great promise, it is still in the experimental stages, and there are concerns about off-target effects, such as unintended genetic changes that could result in harmful consequences.
- Small Molecule Compounds: Another approach involves the use of small molecules to enhance telomere maintenance. These molecules can modulate the activity of telomere-related enzymes and promote telomere extension. For example, compounds like altiratinib, which target telomere maintenance mechanisms, have shown promise in preclinical studies. However, the efficacy and safety of these compounds need to be thoroughly evaluated before they can be considered for human use.
- Stem Cell Therapy: Stem cells possess the ability to divide and generate new cells, making them a powerful tool for regenerative medicine. By manipulating the telomeres of stem cells, it may be possible to rejuvenate tissues and organs. This approach could lead to breakthroughs in regenerative medicine and offer potential treatments for age-related degenerative diseases. However, the ethical implications and safety concerns of using stem cells in this context must be carefully considered.
Ethical and Practical Considerations
While the potential of telomere extension for anti-aging therapies is intriguing, it raises several ethical and practical questions that must be addressed before these technologies can be widely applied.
- Safety and Long-Term Effects: One of the most pressing concerns with telomere extension therapies is the risk of malignancies. Telomeres play a crucial role in preventing uncontrolled cell division, and their extension could inadvertently promote the development of cancer. As cells divide more rapidly, the risk of mutations and genomic instability increases, potentially leading to the formation of tumors. Before telomere extension therapies can be used in humans, extensive research is needed to understand the long-term effects of such interventions, including the potential for cancer and other adverse health outcomes.
- Equitable Access to Anti-Aging Therapies: Another important consideration is equitable access to telomere-based anti-aging therapies. If such treatments become available, it is crucial that they are accessible to all individuals, not just those who can afford them. The potential for exacerbating societal inequalities in health and longevity is a real concern. Those with access to these therapies could experience longer, healthier lives, while those without access may face worsening health disparities. Policymakers and researchers must work together to ensure that these therapies are distributed equitably and that they do not contribute to widening the gap between rich and poor.
- Ethical Implications of Life Extension: The prospect of extending human lifespan raises ethical questions about the nature of aging and the implications of life extension. Some argue that we should accept aging as a natural process and avoid interfering with it, while others believe that extending life could have profound societal benefits, such as increased wisdom, productivity, and overall quality of life. Furthermore, the extension of human lifespan could place strain on resources, healthcare systems, and social structures. The ethical debates surrounding life extension and telomere manipulation are complex and require careful consideration.
- Informed Consent and Regulation: Given the experimental nature of telomere-based therapies, informed consent is a critical issue. Patients must be fully informed of the potential risks and benefits of these therapies before undergoing treatment. Additionally, regulatory agencies must establish guidelines and oversight to ensure the safety and efficacy of telomere extension treatments. The challenge lies in balancing innovation with caution to prevent unintended harm.
Future Directions in Telomere Research
The field of telomere biology is rapidly evolving, with new technologies and discoveries continuously reshaping our understanding of aging. Several exciting future directions in telomere research hold the potential to transform the way we approach aging and age-related diseases.
- CRISPR and Gene Editing: The use of CRISPR-based gene editing technologies is one of the most promising advancements in telomere research. With the ability to precisely target genes associated with telomere maintenance, scientists could potentially develop therapies that selectively extend telomeres or promote telomerase activity. The ability to modify the human genome at such a precise level opens the door to targeted anti-aging therapies that could revolutionize healthcare. However, as mentioned earlier, further research is needed to assess the safety and ethical implications of such interventions.
- Telomere-Based Personalized Medicine: Integrating telomere science with personalized medicine could lead to more tailored approaches for managing aging and age-related diseases. By understanding an individual’s telomere length and the factors that influence it, healthcare providers could develop personalized interventions to optimize aging trajectories. For example, individuals with shorter telomeres may benefit from therapies designed to protect and extend telomere length, while others may need lifestyle interventions to mitigate the effects of telomere attrition.
- Interplay with Other Aging Mechanisms: Aging is a multifaceted process that involves various biological mechanisms, including mitochondrial dysfunction, oxidative stress, and proteostasis. Research into the interplay between telomeres and these other aging mechanisms could uncover synergistic approaches to combat aging. By understanding how telomeres interact with these pathways, scientists may be able to develop more comprehensive anti-aging therapies that target multiple aspects of aging simultaneously.
- Collaboration and Interdisciplinary Research: The future of telomere research will require collaboration across multiple scientific disciplines, including molecular biology, bioinformatics, genetics, and clinical research. Interdisciplinary efforts will be crucial for translating laboratory findings into practical, effective treatments for humans. Collaborative research will also be necessary to address the complex ethical, regulatory, and social issues surrounding telomere manipulation.
Conclusion
Telomeres are central to our understanding of aging and longevity. Their dynamic nature and the interplay between genetic, environmental, and lifestyle factors make them a key target for anti-aging interventions. While the potential for telomere extension therapies is promising, it is important to carefully consider the ethical and practical implications of such technologies. By addressing these concerns and continuing to explore new avenues of research, we may one day unlock the secrets of telomeres and revolutionize the way we approach aging and healthspan.
As we stand on the brink of a new era in anti-aging research, the future of telomere science holds immense potential. From gene editing to personalized medicine, the possibilities are vast. With continued progress and collaboration, scientists may soon develop transformative interventions that redefine the aging experience and offer hope for a longer, healthier, and more vibrant future.
